time:2025-08-28 source:起点锂电
Solid state battery technology continues to attract attention, but rumors about its performance are difficult to distinguish between true and false. Is the energy density higher? Longer cycle life? Can solid electrolytes really suppress lithium dendrites? This article is based on the paper "Industrialization Progress of Polymer Based Solid State Lithium ion Batteries" published by Professor Guo Xin's team in Science Bulletin in August 2025. It breaks down these core cognitive misconceptions one by one, restores the technical truth of solid-state batteries, and takes you to see the real technical landscape and breakthrough path of solid-state batteries.
1、 Do solid-state batteries have higher energy density?
Solid state batteries are often considered to have the advantage of high energy density, but this view is actually not accurate. When using the same positive and negative electrode materials, the energy density of solid-state batteries is often lower than that of liquid batteries due to the higher density of solid electrolytes.
Taking the battery cell with NCM811 as the positive electrode and graphite as the negative electrode as an example for analysis (positive electrode loading>25 mg cm ⁻ ², active material mass ratio of 60%, negative electrode thickness of 10 μ m, mass ratio less than 1%, battery cell size of 90 × 100 mm ², laminated structure including 25 layers of positive electrode and 26 layers of negative electrode). Assuming the use of a 10 μ m thick electrolyte and an E/C ratio (the ratio of electrolyte to battery capacity) of 1 g Ah ⁻¹, and considering that the mass of the separator, current collector, and packaging material is 20%, the energy density of batteries with different types of electrolytes shows significant differences: using liquid electrolytes (such as LiPF ₆+EC+EMC with a specific gravity of 1.1 g cm ⁻¹), oxide electrolytes (such as LLZO with a specific gravity of 3.25 g cm ⁻¹), sulfide electrolytes (such as Li ₆ PS ₅ Cl with a specific gravity of 1.64 g cm ⁻¹), and... The energy densities of polymer electrolytes (such as polyacrylic esters with a specific gravity of 1.3 g cm ⁻¹) for battery cells are calculated to be 370 Wh kg ⁻¹, 125 Wh kg ⁻¹, 248 Wh kg ⁻¹, and 313 Wh kg ⁻¹, respectively( Refer to Table 1), these calculations based on ideal conditions do not represent the true energy density, but only reflect the relative size of energy density. Due to the high density of solid electrolytes, their energy density generally decreases.
However, due to the closest specific gravity between polymer electrolytes and liquid electrolytes, the decrease in energy density of polymer electrolyte batteries is relatively small. The formation of oxide and sulfide electrolytes is difficult, and the thickness usually needs to be at least 50 μ m, making it difficult to thin down to 10 μ m, which leads to further reduction in energy density; The energy density of oxide cells decreases to about 73 Wh kg ⁻¹, while the energy density of sulfide cells decreases to about 178 Wh kg ⁻¹. Compared to liquid batteries, the energy density of sulfide cells decreases by 51.9%, while the energy density of oxide cells decreases by as much as 80.3% (see Table 1). In addition, the conductivity of solid electrolytes is relatively poor, and in order to ensure the required conductivity, a higher electrolyte content is required. Therefore, the E/C ratio may exceed 1 g Ah ⁻¹, which can also lead to a decrease in the energy density of the battery cell.
The effective strategy for improving energy density in solid-state batteries is to use new positive and negative electrode materials. For example, using ultra-thin lithium metal negative electrodes (10 μ m) instead of graphite negative electrodes, and using high-voltage lithium rich manganese based positive electrodes instead of ternary positive electrode materials, can significantly improve the energy density of batteries. Using polyacrylic polymer electrolyte, the thickness of the electrolyte layer is reduced to below 15 μ m, and the E/C ratio is controlled at 1 g Ah ⁻¹. The energy density calculated by the above method can reach 700 Wh kg ⁻¹, significantly higher than that of liquid batteries using NCM811 and graphite.
Therefore, under the same positive and negative electrode system, the energy density of solid-state batteries is usually lower than that of liquid batteries, but using high-voltage positive electrodes and high-capacity negative electrodes (such as high nickel ternary positive electrodes or lithium rich manganese based positive electrodes+metal lithium negative electrodes) can break through the energy density bottleneck and achieve higher performance.
2、 Does solid-state battery have a longer cycle life?
The cycle life of solid-state batteries faces multiple challenges, making it difficult to surpass traditional liquid systems. Due to its inherent brittleness, oxide solid electrolytes are prone to stress accumulation at the interface between the electrode and electrolyte during repeated charging and discharging, causing micro cracks and even interface delamination. This mechanical damage will continue to deteriorate the ion transport path, accelerate the isolation between the active material and electrolyte, and ultimately manifest as a sudden drop in capacity and shortened cycle life. The chemical sensitivity of sulfide electrolytes is exposed during long-term operation. For example, sulfides at the positive electrode interface are easily oxidized and decomposed by high voltage, forming an impedance layer that hinders the diffusion of lithium ions. The continuous reaction between sulfides on the negative electrode side and metallic lithium consumes the electrolyte and causes an increase in porosity, resulting in an exponential decline in the overall performance of the battery. In contrast, liquid electrolytes can dynamically repair interface defects caused by electrode volume changes due to their flow characteristics, and their solvation lithium ion transport mechanism can also buffer local current density fluctuations, thereby maintaining a relatively stable interface chemical environment over thousands of cycles. The lack of dynamic interface adjustment capability and the combined effect of intrinsic material defects in solid-state batteries make it difficult for their cycle life to break through the performance ceiling of liquid system construction.
To address this issue, Solid State Ion Energy Technology (Wuhan) Co., Ltd.'s polymer solid-state battery significantly suppresses side reactions on the positive and negative electrode sides and improves lithium ion conductivity efficiency by creating high ion conductivity and stable SEI and CEI layers. Technical data shows that its solid-state lithium battery (LiNi ₆ ₈Co₀. ₉₇Mn₂. Under the charge and discharge conditions of 25 ℃ and 2.2C/1.0C, the capacity retention rate of the Gr system (₂ ∝ O ₂ | | Gr system) still reached 77.2% after 3500 cycles, exceeding the lifespan performance of conventional liquid batteries. This breakthrough is due to its effective control of positive electrode degradation and interface layer design, which enables the battery to maintain long cycle stability at high energy density, providing important technical support for the industrialization of solid-state batteries.
3、 Do solid-state batteries have faster charging/discharging rates?
The fast charging performance of solid-state batteries is often highly anticipated, but their actual performance is limited by ion conductivity and interfacial behavior, making it difficult to surpass liquid batteries. Although oxide solid electrolytes have high room temperature ionic conductivity (~10 ⁻³ S cm ⁻¹), their rigid interface and high interface impedance limit the dynamics of lithium ion transport, leading to increased polarization at high rates and making it difficult to achieve efficient and fast charging. The ionic conductivity of sulfide solid electrolytes (~10 ⁻² S cm ⁻¹) is close to that of liquid electrolytes, theoretically possessing the potential for fast charging. However, the interface stability issue with the positive and negative electrodes (such as H ₂ S generation) is particularly prominent at high current densities, which not only affects the cycle life but may also pose safety hazards.
Solid State Ion Energy Technology (Wuhan) Co., Ltd. has constructed a new polymer electrolyte system with a gradient Li ⁺ solvation structure through molecular size distribution engineering strategy, accelerating the Li ⁺ decomplexion process, reducing the Li ⁺ migration activation energy to 0.18 eV (a 63% decrease from traditional systems), and achieving an ion conductivity breakthrough of 2.95 × 10 ⁻ ³ S cm ⁻ ¹ at 30 ℃. Polymer based batteries based on this technology can be charged at 4 C rate and discharged at 20 C rate.
4、 Can solid electrolytes inhibit the growth of lithium dendrites?
Solid state batteries are often believed to suppress lithium dendrite growth through the high mechanical strength of solid electrolytes, but this viewpoint needs to be carefully examined in conjunction with the characteristics of different electrolyte systems. The key factors for generating lithium dendrites include the critical current density (CCD) of the electrolyte and the mechanical modulus of the electrolyte. Some argue that when the Young's modulus of the electrolyte is greater than six times that of lithium metal (~6 GPa), it can effectively inhibit dendrite growth through mechanical barriers. However, in actual battery operation, the critical current density directly affects the formation of dendrites: when the current density exceeds CCD, the deposition rate of lithium ions exceeds its diffusion rate, leading to an increase in local lithium ion concentration gradient and inducing dendrite growth. Although oxide solid electrolytes (such as LLZO) have a high mechanical modulus (~150 GPa), much higher than lithium metal, theoretically they can suppress the expansion of lithium dendrites through physical barriers. However, their brittle characteristics and interface contact problems can easily lead to local stress concentration, which may accelerate dendrite penetration. Moreover, at high current densities, local electric field concentration and uneven distribution of interface impedance can still lead to the preferential formation and expansion of dendrites. In addition, the introduction of grain boundaries and micropores during high-temperature sintering process can weaken the uniformity of the electrolyte, leading to uneven distribution of local current density and further exacerbating the growth risk of lithium dendrites. This is similar to the formation mechanism of conductive filaments driven by local electric fields in memristors: in the region of local electric field concentration, the migration rate of ions significantly increases, leading to preferential deposition of ions and the formation of dendritic channels Therefore, oxide electrolytes cannot suppress the growth of lithium dendrites in practical applications.
Sulfide solid electrolytes (such as LPSCl) can achieve tight electrode electrolyte interface contact due to their soft nature, but their low mechanical modulus (~20 GPa) makes it difficult to effectively resist lithium deposition pressure, and dendrites may still penetrate the electrolyte layer under high current density. In addition, the chemical compatibility issues between sulfides and lithium metal, such as interface decomposition, will further exacerbate the interface impedance and uneven distribution of local current density, leading to an increase in the thermodynamic driving force for dendrite growth.
Solid State Ion Energy Technology (Wuhan) Co., Ltd. has achieved synergistic optimization of lithium metal negative electrode/electrolyte interface stability and ion transport kinetics by constructing a dynamic adaptive polymer electrolyte system, introducing self-healing monomers into the polymer electrolyte, endowing the electrolyte with self-healing properties, eliminating uneven Li ⁺ flow caused by interface defects, and suppressing dendrite growth.
5、 Does the development of solid-state batteries follow the path from liquid to semi-solid and then to all solid state?
Some current views suggest that the development of batteries should follow a path from liquid to semi-solid and then to all solid, but this view has logical misconceptions. The development goal of battery technology is to achieve high performance, rather than simply pursuing solidification. Liquid, semi-solid, and all solid state batteries are all means to achieve high-performance batteries, rather than the ultimate goal. Therefore, academia, industry, and government need to re-examine the direction of battery technology development, avoid mistaking means for ends, and focus on achieving high-performance batteries through different technological means.
Compared with liquid batteries, the real advantages of solid-state batteries are high safety and wide operating temperature range. Oxide electrolytes provide high safety due to their non combustible properties. In contrast, although sulfide electrolytes have good ionic conductivity, their flammability reduces safety. At the "China All Solid State Battery Innovation and Development Summit Forum" held on February 15, 2025, BYD Lithium Battery Co., Ltd. released important research results on the safety characteristics of sulfide solid-state batteries. Research has shown that sulfide based solid-state batteries have inherent risks of thermal runaway, which can occur irreversibly under extreme working conditions. Thermal runaway is characterized by intense processes, high temperatures, fast speeds, and high overpressure. This study provides important safety assessment basis for the selection of all solid state battery technology routes, and suggests that the industry must establish multidimensional thermal management solutions while pursuing high energy density.
In addition, Yersak et al.'s study confirmed that sulfide electrolytes (such as LPSCl) decompose and release flammable sulfur vapor under thermal abuse conditions (>150 ℃), which can be ignited upon contact with air, accompanied by bright flames (often observed as blue purple) and possible hot particle jets, followed by the formation of ashing products on the material surface. These ash contents contain oxidation products (such as Li ∝ PO ₄, Li ₂ SO ₄, etc.), indicating that this process is a strong oxidation reaction. This characteristic fundamentally distinguishes it from non combustible oxides (such as LLZO) and flame-retardant polymer electrolytes. Polymer electrolytes typically have flame retardant properties, which also ensure the safety of batteries. In addition, solid electrolytes have significant advantages in operating over a wide temperature range. Liquid electrolytes evaporate at high temperatures and solidify at low temperatures, while solid electrolytes can maintain stable performance over a wide temperature range. Therefore, the advantages of solid-state batteries need to be evaluated based on more comprehensive and in-depth research.
In summary, the development of battery technology should focus on high-performance goals rather than solid-state processes. The core advantages of solid-state batteries are high safety (non combustible oxide/polymer electrolytes) and wide temperature range adaptability, but they need to break through the bottleneck of ion conductivity and balance material properties (such as sulfide flammability), and achieve breakthroughs through multi technology path collaborative optimization rather than single form iteration.
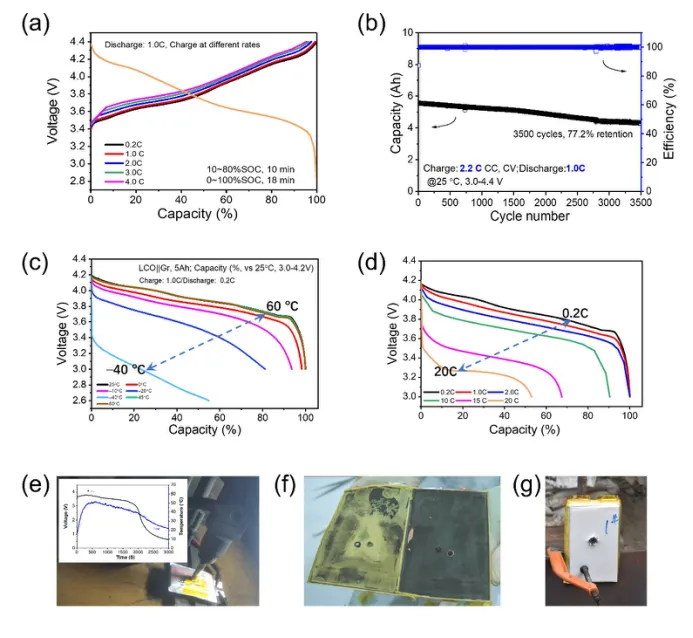
Figure 1 Performance of Polymer Solid State Lithium Battery Developed by Solid State Ion Energy Technology (Wuhan) Co., Ltd. (a) LiNi₆. ₈Co₀. ₉₇Mn₂. The capacity voltage curve of a Gr battery charged at 0.2 C to 4 C rates shows a capacity retention rate of 95.52% at 0.2 C when charged at 4 C rates. (b) LiNi₆. ₈Co₀. ₉₇Mn₂. The long-term cycling performance of the Gr battery at room temperature showed a capacity retention rate of 77.2% after 3500 cycles. (c) Capacity voltage curve of LiMnO ₂+LiCoO ₂ | | Gr battery at -40~60 ° C. (d) Capacity voltage curve of LiMnO ₂+LiCoO ₂ | | Gr battery discharged at 0.2-20 C rate. (e) NCM613 | | Gr solid-state battery 12mm steel needle single needle puncture test. (f) Internal unfolded diagram of solid-state battery. (g) Front view of solid-state battery shooting test sample.
Solid State Ion Energy Technology (Wuhan) Co., Ltd. has made technological breakthroughs in the field of solid-state batteries and actively laid out the industrialization process. The company has developed a LiNi ₆ technology to address the range anxiety issue of new energy vehicle power batteries ₈Co₀. ₉₇Mn₂. A fast charging polymer solid-state lithium battery with graphite (Gr) as the negative electrode and ₂ ∝ O ₂ as the positive electrode material, has an energy density of 260Wh kg ⁻¹. The maximum charging rate of the battery reaches 4.0 C, with a capacity retention rate of over 95% compared to 0.2 C rate (see Figure 1 (a)), and the capacity retention rate still reaches 77.2% after 3500 charge and discharge cycles (see Figure 1 (b)), demonstrating excellent fast charging performance and cycle stability. When using NCM811 positive electrode and silicon carbon negative electrode (40% Si), the energy density of the battery cell can be increased to 320 Wh kg ⁻¹;; when using NCM811 positive electrode and lithium metal negative electrode, the energy density of the battery cell can be increased to 450 Wh kg ⁻¹, providing a more efficient and reliable power solution for new energy vehicles.
Polymer solid-state lithium batteries for consumer electronics applications also perform well. The battery, which uses LiMnO ₂ and LiCoO ₂ composite positive electrodes and Gr negative electrodes, has a capacity retention rate of 55.6% at an extreme low temperature of -40 ℃ (see Figure 1 (c)), a capacity retention rate of 89.5% after 150 cycles at 100 ℃, and a capacity retention rate of 52% at a high rate discharge of 20 ℃ (see Figure 1 (d)). In terms of safety, this solid-state battery system has passed multiple rigorous tests. In the needle puncture test, the NCM613 | | Gr battery was subjected to a single needle puncture with a 12mm steel needle (see Figure 1 (e)), and only heated to 50 ℃ without smoking or ignition, demonstrating extremely high safety. In addition, the disassembly image of the solid-state battery confirms that there is no liquid electrolyte inside (see Figure 1 (f)). After a rigorous shooting test (withstanding a 5.8mm regular bullet), the battery sample remained stable without smoke, fire, or explosion (see Figure 1 (g)).